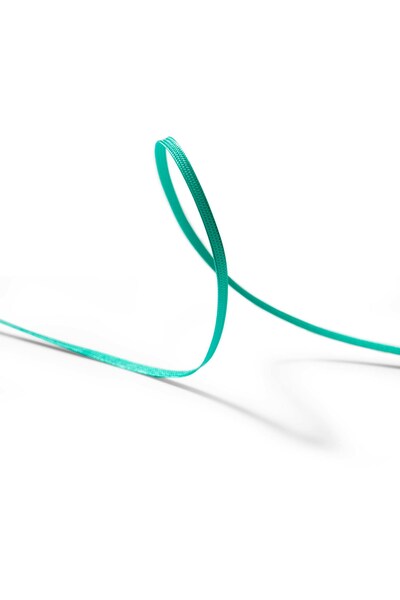
Riverpoint Medical, a pioneer in advanced surgical fiber-related technologies, proudly announces a groundbreaking achievement with the introduction of the first human implantable green UHMWPE suture, continuing a radiant journey in medical innovation.
PORTLAND, Ore., Feb. 13, 2024 /PRNewswire-PRWeb/ -- Riverpoint Medical, a pioneer in advanced surgical fiber-related technologies, proudly announces a groundbreaking achievement with the introduction of the first human implantable green UHMWPE suture, continuing a radiant journey in medical innovation. The company is pleased to collaborate with DSM Biomedical, a renowned global science-based company, to bring this cutting-edge technology to the forefront of medical solutions.
Building on the success of securing nine 510(k) clearances and three MDR approvals over the past 24 months, Riverpoint Medical continues to lead in the medical device industry. This regulatory milestone underscores the company's unwavering commitment to delivering state-of-the-art medical solutions while adhering to rigorous standards.
Edwin Anderson, Riverpoint's VP of Regulatory and R&D, emphasizes the significance of this achievement, stating, "The introduction of the first human implantable green UHMWPE suture aligns perfectly with our commitment to rapid innovation. It not only establishes a new standard in surgical fiber technology but also exemplifies our dedication to surpassing regulatory requirements for complex medical textiles."
This revolutionary green UHMWPE suture addresses the challenges faced in complicated surgeries that necessitate the use of multiple fibers and colors. The distinctive green color provides a visual differentiator, enhancing precision and efficiency in the operating room. This breakthrough is poised to revolutionize surgical procedures, offering surgeons a unique tool to improve patient outcomes.
Riverpoint Medical invites the medical community to explore its latest capabilities and innovations at the 2024 AAOS meeting in San Francisco. Interested parties are encouraged to visit Riverpoint Medical's booth number 2059 for comprehensive information, engagement, and a firsthand look at the groundbreaking green UHMWPE suture.
About Riverpoint Medical
Riverpoint Medical is a leading developer, designer and manufacturer of medical devices focused on advanced surgical fiber related technologies. Riverpoint has a strong history of industry leading product innovation and development across bioabsorbable suture and high strength suture-based implants and constructs. Riverpoint employs a business-to-business model through multiple channels and enjoys partnerships with many of the top Global Medtech Companies in the world.
About DSM Biomedical
As the world's unrivaled biomaterials expert and committed partner in driving sustainable innovation in healthcare, DSM Biomedical, a subsidiary of dsm-firmenich, is at the forefront of biomaterial science and process innovation. The company's technologies and support are recognized for their unmatched quality, reliability, and performance in multiple therapeutic areas worldwide. To learn more, visit DSMBiomedical.com.
For more information about Riverpoint Medical, please contact Customer Service at [email protected] or call (503) 517-8001. Visit our website at for the latest updates and details.
Media Contact
Customer Service, Riverpoint Medical, 1 5035178001, [email protected]
SOURCE Riverpoint Medical


Share this article