The top medical priority for the Ukrainian military in the US non-lethal aid package is Prytime Medical Device's pREBOA-PRO™ catheter because it buys time for their surgical teams to transport bleeding soldiers to safety
SAN ANTONIO, Feb. 6, 2024 /PRNewswire-PRWeb/ -- Prytime Medical Devices Inc, The REBOA Company™, the leading innovator and global provider of lifesaving REBOA products for minimally invasive, endovascular hemorrhage control, announced the donation of significant non-lethal medical products and training valued over $1,750,000 to Ukraine.
"The pREBOA-PRO™ catheter is a true, life-saving intervention," said Colonel Konstantine Gumeniuk, Chief Surgeon of the Ukrainian Armed Forces. "The key challenge my field surgeons face is transporting bleeding soldiers to a safe field hospital where we can save their lives. The pREBOA-PRO™ catheter helps Ukraine do this. That's why it has been my top priority to get REBOA included in the non-lethal medical aid package from the United States. Just like bandages and tourniquets, we need pREBOA-PRO™ catheters to be part of our standard surgical tool kit," continued Colonel Gumeniuk.
"No one should bleed to death, and the lessons we are learning from the Ukrainian surgeons are helping save lives back here in the US today," said David Spencer, CEO of Prytime Medical Devices, Inc. "We know from our US physicians that the pREBOA-PRO™ catheter buys time to treat patients. The use in Ukraine shows that it can also be used to safely transport the bleeding wounded off the battlefield. Complete occlusion REBOA catheters have a much shorter time limit for use which prevents them from facilitating transport. The pREBOA-PRO™ catheter was designed for extended occlusion times which is giving the Ukrainians time to transport the bleeding wounded to safety," continued Spencer.
Humanitarian Medical Support for Ukraine in 2023
Prytime Medical's humanitarian aid to Ukraine includes the following.
Training support
- 500+ physicians trained, including civilian, military, and Ukrainian Intelligence service personnel
- 22 hospitals trained in 10 Ukrainian cities and classified far-forward locations
- 7 REBOA training centers established
- 70+ hand-on training hours using a pulsatile simulator
- 8 in-country, in-person training trips
Product support
- 150 Prytime catheters, both ER-REBOA™ PLUS and pREBOA-PRO™
- 25 REBOA training catheters
- 130 REBOA convenience kits
- 130 COMPASS® blood pressure monitors
- 7 ARTI™ Simulator Systems (pulsatile training simulators)
Saving Lives in Ukraine Saves Lives in the United States
"It's an honor and a privilege to help the Ukrainian people in their time of need," continued Spencer. "Helping Ukraine matters in three key ways
1. Save lives and providing humanitarian aid to our ally, Ukraine
2. Accelerate our learning of how to save the lives of US and NATO soldiers in future conflicts
3. Accelerate our learning of how to save civilian lives in the US today
We are also helping Americans. Some people feel that supporting Ukraine doesn't help America, but I disagree. Saving lives in one part of the world helps us in other parts of the world. It's all connected. Prytime has the scale and network to share these learnings across the globe," concluded Spencer.
About the pREBOA-PRO™ Catheter
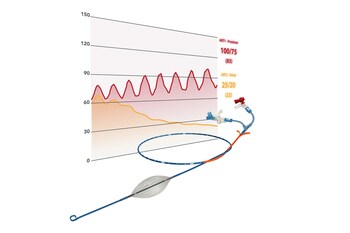
Time is the enemy of trauma surgeons. A landmark study(1) in 2018 showed that it can take bleeding patients over 2 hours to get definitive care after they finally arrive at the hospital. These hemodynamically unstable patients are typically so sick that their need for REBOA exceeds the standard 30 minutes usage in aortic Zone 1. Partial REBOA, or partial occlusion of the aorta, allows the viscera to be sufficiently perfused during the REBOA procedure.
The pREBOA-PRO™ catheter is an endovascular device designed to occlude the aorta to prevent patients from bleeding to death from injuries that can't use a tourniquet to stop the bleeding. As the first catheter designed to deliver True Partial REBOA™, the pREBOA-PRO™ catheter breaks the previous 30-minute Zone 1 barrier and buys time – a resource physicians don't have when their patients are at the risk of exsanguinating. With the ability to precisely titrate the level of occlusion and distal blood flow needed for the patient – like a dimmer switch™ – physicians can safely extend aortic occlusion time while reducing the risk of ischemic injury.
- In North America, it buys physicians time to treat
- In Ukraine, it buys physicians time to transport patients to a safe medical site
A recent summary of published literature highlights three key clinical benefits for the pREBOA-PRO™ catheter:
1. Buying time for extended safe occlusion times beyond 30 minutes 2, 3, 4, 5, 6, 7
2. Expanding treatment options 6, 7, 8 including more CT scans and less blood use
3. Reducing serious complications due to Acute Kidney Injury (AKI) 10, 11, 12, 13, 14
The pREBOA-PRO™ catheter is currently in limited market release at 14 trauma centers in the US and Canada. There have been over 500 civilian patient uses in North America to date.
About Prytime Medical Devices, Inc.
Prytime Medical™ Devices, Inc. is an innovative medical device company that designs, develops, and commercializes minimally invasive solutions for hemorrhage control. The underlying intellectual property for REBOA was conceived based on lessons learned in war. Our latest innovations, the industry leading ER-REBOA PLUS™, and the innovative pREBOA-PRO™ Partial REBOA Catheter, enable torso hemorrhage control with more controlled resuscitation in a much wider range of clinical scenarios. The pREBOA-PRO™ catheter is the first and only FDA-cleared REBOA catheter designed specifically for True Partial REBOA™. More information can be found at
1 J.B. Holcomb, Transport Time and Preoperating Room Hemostatic Interventions Are Important: Improving Outcomes After Severe Truncal Injury, Crit Care Med 46(3) (2018) 447-453.
2 Prytime Medical Centers' of Excellence. Data on file.
3 Polcz et al., (2022) J Surg Res. Based on preclinical data. Clinical results in humans are unknown. Based on preclinical data. Clinical results in humans are unknown.
4 Edwards et al., (2022) J Am Coll Surg. Based on preclinical data. Clinical results in humans are unknown.
5 Necsoiu et al., (2021) Shock. Based on preclinical data. Clinical results in humans are unknown.
6 Kemp et al., (2021) J Trauma Acute Care Surg. Based on preclinical data. Clinical results in humans are unknown.
7 Ho et al., (2023) J Trauma Acute Care Surg. Based on preclinical data. Clinical results in humans are unknown.
8 Nguyen et al., (2023), Maximizing patient care: Partial REBOA enables increased use of CT imaging and endovascular hemorrhage control, AAST poster
9 Nguyen et el., (2024), pREBOA vs ER-REBOA Impact on Blood Utilization and Resuscitation Requirements: A Pilot Analysis, EAST presentation
10 Russo et al., (2020) J Trauma Acute Care Surg.
11 Gomez et al., (2023) J Trauma Acute Care Surg.
12 Ronaldi et al., (2021) Shock. Based on preclinical data. Clinical results in humans are unknown.
13 Madurska et al., (2021) Eur J Trauma Emerg Surg.
14 Hunt et al., (2023) The American Surgeon
Media Contact
Jeffrey Jung, Prytime Medical Devices, Inc, 2105441752, [email protected], prytimemedical.com
SOURCE Prytime Medical Devices, Inc








Share this article