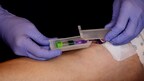
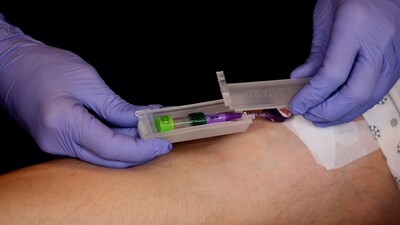
The Infusion Nurses' Society Infusion Therapy Standards of Practice Version 9 was recently released. Two new Standards, Standard 37 and 66, address the care of patients who inject drugs (PWID) inside and outside of the hospital. PICC Guard is the only FDA-cleared tamper-evident device available to meet the site protection requirements for Standard 37. It can assist home care agencies in managing PWID and other high-risk patients, allowing them to complete their antibiotic treatment at home now as recommended in Standard 66.
SHEBOYGAN, Wis., March 11, 2024 /PRNewswire-PRWeb/ -- The (INS) recently released Version 9 of . This update included two new Standards, Standard 37 Site Protection and Joint Stabilization and Standard 66 Home Infusion Therapy. These two Standards address a high-risk population in healthcare: patients who inject drugs (PWID). The opioid epidemic continues to plague our country. It has created a patient population that requires expensive, long-term infusion therapy to treat illnesses such as endocarditis and osteomyelitis. Typical treatment for these disease states involves six weeks of intravenous antibiotic therapy once or twice a day. However, due to their history, clinicians have not felt comfortable placing the typical infusion catheter, a peripherally inserted central catheter (PICC), in this group of patients.
The new INS Standard 37.1 states, "Site protection methods protect vascular access devices (VADs) or VAD sites from patient manipulation, inadvertent dislodgement, and contaminant exposure." Also: "Use of tamper-evident procedures/products is considered when patients are at risk for VAD misuse, including hospitalized persons who inject drugs (PWID). While studies report that misuse of VADs is low in PWID who receive home infusion therapy, some programs use a tamper-evident procedure/product at the catheter hub as a form of site protection (refer to Standard 66, Home Infusion Therapy)." PICC Guard is the only FDA-cleared tamper-evident, tamper-resistant device that can protect catheters from exposure to contaminants. This provides the opportunity to monitor and discharge high-risk patients, such as patients with a history of IV Drug abuse, with their VAD in place.
Katie Justus, RN, the President of PICC Guard, LLC, stated, "As a small business whose mission is to improve the standard of care for patients, it is exciting to see the new additions to the INS Infusion Therapy Standards of Practice. Hospitalized patients with a history of substance use are at risk for using illegal drugs while hospitalized, which could lead to infection, overdose, or other complications. PICC Guard can reduce the cost of care for these patients by eliminating the need for a sitter and moving them to outpatient care sooner."
The new INS Standard 66 addresses various aspects of home infusion therapy, including home care for PWID—Standard 66 supports caring for these high-risk patients at home when the environment is supportive. With PICC Guard, the clinicians and caregivers managing the patient's treatment can have confidence that the patient's VAD is safe to use while allowing the patient the freedom and comfort of being at home rather than hospitalized until the end of therapy. Jesse Justus, a Board Certified Emergency Medicine physician, states, "The new additions to the INS Infusion Therapy Standards of Practice are inspiring. These bring awareness to a problem prevalent in healthcare for years. With these new Standards, we will be able to create a change in the culture of treating patients with a history of substance use.
About , LLC
A privately held company based in the United States, PICC Guard, LLC believes in transforming lives through medical innovation. The co-founders are seasoned medical professionals emphasizing accountability, quality, and improving our communities through innovative medical technology. The PICC Guard device was created to fill a void identified in caring for high-risk patients such as patients with a history of substance use, confused, incarcerated, or pediatric patients. Designed to cover and protect the access points of an IV catheter, the PICC Guard can reduce the risk of CLABSI, shorten the length of stay, and lower the risk of accidental overdose. The first device to be cleared by the FDA is the market leader in IV catheter tamper-evidence and access protection.
About , LLC
A privately held company in the United States, RyMed Technologies, LLC is committed to improving patient outcomes related to catheter-related bloodstream infection prevention and catheter occlusion reduction by providing product solutions with fail-safe engineering design technologies. RyMed is the market leader in antimicrobial needleless connectors. PICC Guard protects a high-risk population from complications in any patient safety initiative. RyMed is proud to partner with PICC Guard, LLC.
Media Contact
Katie Justice, RN, President, PICCGuard, LLC, 1 918-519-2563, [email protected],
Todd Adamson, Director of Sales, RyMed Technologies, LLC, 1 615-790-8093, [email protected],
,
SOURCE PICCGuard, LLC

Share this article